Журнал "Гастроэнтерология" Том 55, №1, 2021
Вернуться к номеру
Клініко-вегетативні порушення, адаптаційні можливості та стресостійкість у хворих на передракові стани шлунка в умовах коморбідності з патологією щитоподібної залози
Авторы: L.M. Mosiychuk, O.M. Shevtsova, O.P. Petishko
State Institution “Institute of Gastroenterology of the National Academy of Medical Sciences of Ukraine”,
Dnipro, Ukraine
Рубрики: Гастроэнтерология
Разделы: Клинические исследования
Версия для печати
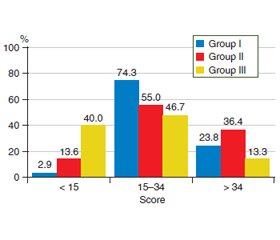
Актуальність. Важливим аспектом перебігу передракових станів шлунка є коморбідність із патологією щитоподібної залози. На сьогодні серед дослідників досі немає єдиної думки щодо участі вегетативної нервової системи у забезпеченні реактивності організму і формуванні адаптаційних процесів у хворих з атрофічним гастритом, поєднаним з тиреоїдитом. Мета дослідження: визначити особливості клінічних проявів, вегетативної дисфункції та показників варіабельності серцевого ритму у хворих iз передраковими станами шлунка в умовах коморбідної патології щитоподібної залози. Матеріали та методи. У дослідження включені 72 пацієнти з хронічним атрофічним гастритом, які були розподілені на 3 групи залежно вiд змін у структурі щитоподібної залози: І групу становили 35 осіб із вузловими змінами, ІІ — 22 із дифузними, ІІІ — 15 осіб без будь-яких змін. Для вивчення клінічної картини в обстежених хворих проведено опитування з використанням Шкали оцінки симптомів з боку шлунково-кишкового тракту. Для виявлення ознак вегетативних порушень використовували опитувальник О.М. Вейна. Оцінку адаптаційних можливостей проводили за допомогою системи Precise на кардіографі CONTEC 8000GW з програмним забезпеченням для аналізу варіабельності серцевого ритму. Результати. У пацієнтів із коморбідною патологією найчастіше виявлялися симптоми абдомінального больового синдрому та диспептичного синдрому незалежно від структурних змін щитоподібної залози, а діарейний синдром в І групі відмічали в 1,9 раза рідше порівняно з ІІ групою (р = 0,02) та в 1,8 раза порівняно з ІІІ групою (р > 0,05). Встановлено високу частоту вегетативної дисфункції у хворих із передраковими станами шлунка — 83,3 %. При цьому пацієнти з вузловими змінами в щитоподібній залозі достатньо часто скаржилися на зниження працездатності, швидку втому (68,6 %) та порушення сну (65,7 %). У хворих із дифузними змінами в щитоподібній залозі часто спостерігали оніміння пальців рук, стоп (68,2 %), схильність до почервоніння обличчя та підвищену пітливість (63,6 %). Визначено кореляцію кількості балів за опитувальником О.М. Вейна з інтенсивністю таких синдромів, як абдомінальний біль (r = 0,53; р = 0,030), діарейний синдром (r = 0,58; р = 0,012), диспептичний синдром (r = 0,44; р = 0,029). Аналіз стресостійкості показав, що 33,3 % пацієнтів iз вузловими змінами в щитоподібній залозі та 28,6 % iз дифузними мали тривожний синдром, у той час як серед осiб без патології щитоподібної залози тривожність не виявлено в жодному випадку. Висновки. За даними системи Precise, половина пацієнтів iз передраковими станами шлунка в умовах коморбідної патології щитоподібної залози мають підвищений серцево-судинний ризик на фоні збільшення парасимпатичної регуляції вегетативної нервової системи, а третина хворих — зрив/порушення адаптації, тривожний синдром та виснаженість симпатичного та парасимпатичного компонентів вегетативної нервової системи. Виявлені зв’язки свідчать про значний внесок вегетативних порушень у формування клінічної симптоматики в пацієнтів iз коморбідною патологією шлунка та щитоподібної залози.
Background. An important aspect of the course of precancerous conditions of the stomach is comorbidity with thyroid pathology. To date, there is no consensus among researchers regarding the involvement of the autonomic nervous system in ensuring the reactivity of the body and the formation of adaptive processes in patients with atrophic gastritis combined with thyroiditis. The purpose was to determine the features of clinical manifestations, autonomic status and heart rate variability in patients with precancerous conditions of the stomach in comorbid thyroid pathology. Materials and methods. The study included 72 patients with chronic atrophic gastritis, who were divided into 3 groups depending on the changes in the structure of the thyroid gland: group I consisted of 35 people with nodular changes, II — 22 with diffuse changes, III — 15 people without any changes. To study the clinical picture in the examined patients, a survey was conducted using the Gastrointestinal Symptom Rating Scale. To identify signs of autonomic disorders, we used the questionnaire of O.M. Vein. Adaptive capacity was assessed using Precise system on a CONTEC 8000GW cardiograph with software that analyzes heart rate variability. Results. In patients with comorbid pathology, symptoms of abdominal pain syndrome and dyspeptic syndrome were often revealed regardless of structural changes in the thyroid gland; the frequency of diarrheal syndrome in group I was 1.9 times lower than in group II (p = 0.02) and 1.8 times compared with group III (p > 0.05). A high frequency of autonomic dysfunction (83.3 %) was detected in patients with precancerous conditions of the stomach. At the same time, individuals with nodular changes in the thyroid gland quite often complained of decreased performance, rapid fatigue (68.6 %) and sleep disturbances (65.7 %). Patients with diffuse changes in the thyroid gland had numbness of the fingers and toes (68.2 %), a tendency to facial redness and increased sweating (63.6 %). Correlations were determined between the score on Vein's questionnaire and the severity of syndromes such as abdominal pain (r = 0.53; p = 0.030), diarrheal syndrome (r = 0.58; p = 0.012), dyspeptic syndrome (r = 0.44, p = 0.029). Analysis of stress resistance showed that 33.3 % of patients with nodular changes in the thyroid gland and 28.6 % of those with diffuse changes had anxiety syndrome, while people without thyroid pathology had no anxiety. Conclusions. According to Precise system data, half of patients with precancerous conditions of the stomach in thyroid comorbidity have an increased cardiovascular risk against the background of an increase in parasympathetic regulation of the autonomic nervous system, and one-third of patients have a breakdown/disorder of adaptation, anxiety syndrome and depletion of the sympathetic and parasympathetic components of the autonomic nervous system. The revealed correlations indicate a significant contribution of autonomic disorders to the formation of clinical symptoms in patients with comorbid pathology of the stomach and thyroid gland.
вегетативна дисфункція; адаптаційні можливості; стресостійкість; передракові стани шлунка; патологія щитоподiбної залози
autonomic dysfunction; adaptive capabilities; stress resistance; precancerous conditions of the stomach; thyroid pathology
Introduction
Material and methods
Results and discussion
/15.jpg)
Conclusions
- Castoro С. et al. Association of autoimmune thyroid diseases, chronic atrophic gastritis and gastric carcinoid: experience from a single institution. J. Endocrinol. Invest. 2016. Vol. 39(7). P. 779-784.
- Бакулин И.Г. и др. Коморбидный пациент в гастроэнтерологии: индивидуальный поход. Рациональная фармакотерапия в кардиологии. 2018. Т. 14. № 1. С. 65-69.
- Мосійчук Л.М. та ін. Оцінка коморбідного статусу й структурних змін щитоподібної залози у хворих на хронічний метапластичний атрофічний гастрит: огляд і власні дослідження. Гастроентерологія. 2020. Т. 54. № 3. С. 155-171.
- Goemann I.M. et al. Role of thyroid hormones in the neoplastic process: an overview. Endocr. Relat. Cancer. 2017. Vol. 24(11). P. R367-R385.
- Сорокман Т.В. та ін. Захворювання органів шлунково-кишкового тракту при супутній патології щитоподібної залози (огляд літератури). Здоровье ребенка. 2019. Т. 14. № 1. С. 2-9.
- Li Y., Xia R., Zhang B., Li C. Chronic atrophic gastritis: a review. J. Environ. Pathol. Toxicol. Oncol. 2018. Vol. 37(3). P. 241-259.
- Annibale B., Esposito G., Lahner E. A current clinical overview of atrophic gastritis. Expert Rev. Gastroenterol. Hepatol. 2020. Vol. 14(2). P. 93-102.
- Rodriguez-Castro K.I. et al. Clinical manifestations of chronic atrophic gastritis. Acta Biomed. 2018. Vol. 89(8-S). P. 88-92.
- Shin S.Y. et al. Chronic atrophic gastritis and intestinal metaplasia surrounding diffuse-type gastric cancer: Are they just bystanders in the process of carcinogenesis? PLoS One. 2019. Vol. 14(12). P. e0226427.
- Wehrwein E.A., Orer H.S., Barman S.M. Overview of the anatomy, physiology, and pharmacology of the autonomic nervous system. Compr. Physiol. 2016. Vol. 6(3). P. 1239-1278.
- Gibbons C.H. Basics of autonomic nervous system function. Handb. Clin. Neurol. 2019. № 160. P. 407-418.
- Петраш М.Д., Гребенников В.А. Особенности вегетативной регуляции при воздействии повседневных стрессоров: возрастно-половой аспект. Мир науки. 2018. № 6. https://mir-nauki.com/PDF/64PSMN618.pdf.
- Чернин В.В., Джулай Г.С. Клинико-патогенетические варианты хронического гастрита. Терапевтический архив. 2004. Т. 79. № 2. С. 22-27.
- Воронич С.М., Павликівська Б.М., Воронич-Семченко Н.М. Фізіологічні аспекти аналізу показників мінливості серцевого ритму у підлітків із прихованим гіпотиреозом. Фізіологічний журнал. 2010. Т. 56. № 5. С. 53-61.

/13_2.jpg)
/13.jpg)
/14.jpg)
/14_2.jpg)
/14_3.jpg)